Realities, challenges and development levers
Catégorie : Général
Etudes AHA 2021
Etudes ESC 2021
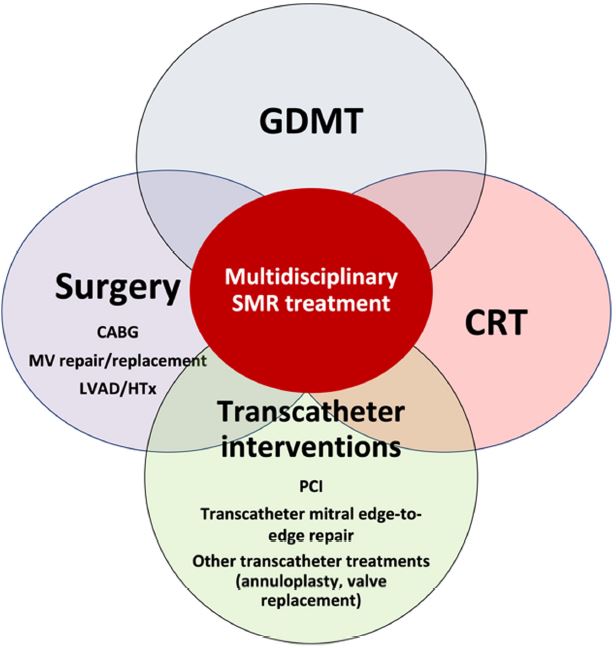
The management of secondary mitral regurgitation in patients with heart failure: a joint position statement from the Heart Failure Association (HFA), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), and European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC
Les conséquences du confinement sur les maladies cardiovasculaires
The year in cardiovascular medicine 2020, articles sélectionnés par Dr Tahar El Kandoussi. El Hoceima
Peripartum management of hypertension: a position paper of the ESC Council on Hypertension and the European Society of Hypertension
Abstract :
Hypertensive disorders are the most common medical complications in the peripartum period associated with a substantial increase in morbidity and mortality. Hypertension in the peripartum period may be due to the continuation of pre-existing or gestational hypertension, de novo development of pre-eclampsia or it may be also induced by some drugs used for analgesia or suppression of postpartum haemorrhage. Women with severe hypertension and hypertensive emergencies are at high risk of life-threatening complications, therefore, despite the lack of evidence-based data, based on expert opinion, antihypertensive treatment is recommended. Labetalol intravenously and methyldopa orally are then the two most frequently used drugs. Short-acting oral nifedipine is suggested to be used only if other drugs or iv access are not available. Induction of labour is associated with improved maternal outcome and should be advised for women with gestational hypertension or mild pre-eclampsia at 37 weeks’ gestation. This position paper provides the first interdisciplinary approach to the management of hypertension in the peripartum period based on the best available evidence and expert consensus.
Quoi de neuf en insuffisance cardiaque?
Les inégalités thérapeutiques dans la prise en charge des insuffisances cardiaques à fraction d’éjection réduite (ICFEr) et préservée (ICFEp) continuent à se creuser. En effet, ces 12 derniers mois, deux nouvelles classes thérapeutiques [1, 2], les inhibiteurs sélectifs du cotransporteur 2 du sodium- glucose (SGLT2) et les stimulateurs de la guanylate cyclase soluble (GCs), se sont révélées efficaces dans le traite- ment de l’ICFEr. Ces résultats amènent à réfléchir sur la place respective de ces traitements dans l’algorithme théra- peutique de cette maladie qui semblait gravé dans le marbre depuis les dernières recommandations de 2016 [3], alors que le plus grand essai thérapeutique jamais réalisé en matière de traitement de l’ICFEp, l’étude PARAGON-HF avec le sacubitril-valsartan [4] s’est révélé une nouvelle fois neutre, soulignant les limites du traitement de cette patholo- gie pléiotrope, complexe, qui intéresse pourtant 50 % de nos patients.